
News - Page 1
Fifth International Newborn Screening Day

June 30, 2025
This year marks the fifth anniversary of Newborn Screening (NBS) day, an event celebrated annually on June 28 to coincide with the birthday of Robert Guthrie, the American doctor who developed the first NBS test.
The International Neonatal Screening Day (INSD) campaign is organized by Screen4Rare, a multi-stakeholder platform launched by several partners, including the International Society for Neonatal Screening (ISNS), the International Patient Organisation for Primary Immunodeficiencies (IPOPI) and the European Society for Immunodeficiencies (ESID).
Read moreMedComms Day 2025

June 25, 2025
Today is MedComms Day, a global celebration that highlights the key role played by the Medical communication community in healthcare and medical research. Today we are celebrating the achievements of our team of MedComms professionals and their contributions to medical and scientific writing.
We assist many clients in the writing of various documents and materials, intended for several target audiences and covering diverse medical specialties.
Read moreInternational Clinical Trials Day 2025

May 20, 2025
Medical writing −our core business activity− is a key component of clinical research. Clinical research refers to all research carried out on humans. To raise awareness of the importance of this type of research, International Clinical Trials Day is celebrated worldwide each year on the anniversary of research initiated by James Lind on May 20, 1747, a study which is now considered to be the first ever clinical trial.
Read moreBrain Awareness Week 2025

March 10, 2025
Brain Awareness Week is taking place from the 10th to the 16th of March 2025!
Brain Awareness Week is a global campaign to raise awareness of the importance and benefits of brain research. The campaign was first launched by the Dana Foundation in the United States in 1996, and has since become a worldwide celebration of brain science.
Read moreRare Disease Day 2025

February 28, 2025
We support Rare Disease Day (RDD) 2025!
Since 2008, the last day of February has been dedicated to raising public awareness of rare diseases. Each rare disease affects less than 1 in 2,000 people, but because nearly 7,000 rare diseases have been identified so far, altogether these diseases concern more than 300 million people worldwide (excluding caregivers)!
The European campaign is coordinated by EURORDIS-Rare Diseases Europe. This year, the slogan is “More than you can imagine“.
Read moreWorld Cancer Day 2025

February 4, 2025
February 4, 2025, marks World Cancer Day!
This annual international awareness day, led by the Union for International Cancer Control (UICC) since 2000, helps to unite and support the cancer community and mobilize individuals, organizations and governments to ensure that cancer prevention, reduction of the global cancer burden, and equitable access to cancer treatment and care, remain a top priority for global health.
Read moreICMJE 2025 Recommendations Update

January 31, 2025
The International Committee of Medical Journal Editors (ICMJE) is a working group of editors from general medical journals*. Each year, its members update the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals.
Each medical writer in our team follows the recommendations developed by the ICMJE to write manuscripts for peer-reviewed journals, in parallel with the appropriate study-specific EQUATOR (Enhancing the QUALITY and Transparency Of health Research) guidelines and the instructions to authors for the target journal. The January 2025 update of the ICMJE recommendations has just been published.
Read more2024 Update of the Declaration of Helsinki

January 23, 2025
The Declaration of Helsinki (DoH) is a “statement of ethical principles for medical research involving human participants”. Adopted for the first time in June 1964 in the Finnish capital, it was amended for the 9th time during the 75th General Assembly of the World Medical Association (WMA), which again returned to Helsinki in October 2024. The English version of this latest revision was published on the WMA website on December 13, 2024.
Our medical writing team assists clients in the drafting of the regulatory and clinical documentation necessary for the implementation of medical research, and in the writing of manuscripts for peer-reviewed journals reporting the research results. It was therefore important for us to share this news!
We analyzed the changes made in the 2024 version, 11 years after the previous update.
Read moreTeam meeting in Gironde

October 25, 2024
This year, the annual Santé Active Edition – Synergy Pharm team seminar took place in the area around Bordeaux!
The seminar provided an excellent opportunity to discuss current and future medical and scientific writing projects.
Read moreInvolvement of the cutaneous microbiota in acne
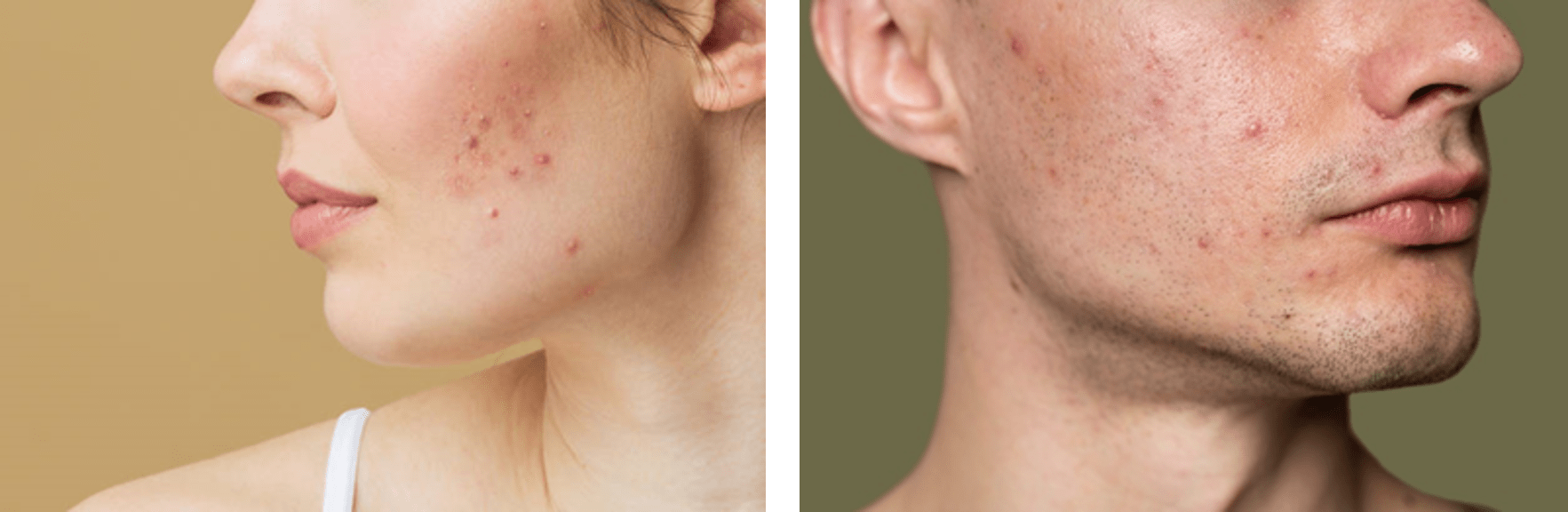
September 13, 2024
Acne vulgaris mainly affects teenagers, but it can also affect adults as either a persistent or a late-onset disorder. In addition to having an aesthetic impact, this skin disorder also affects the emotional wellbeing of affected individuals. The development of acne lesions results from various factors, with recent studies showing that dysregulation of the skin microbiota plays a major role.
Read more